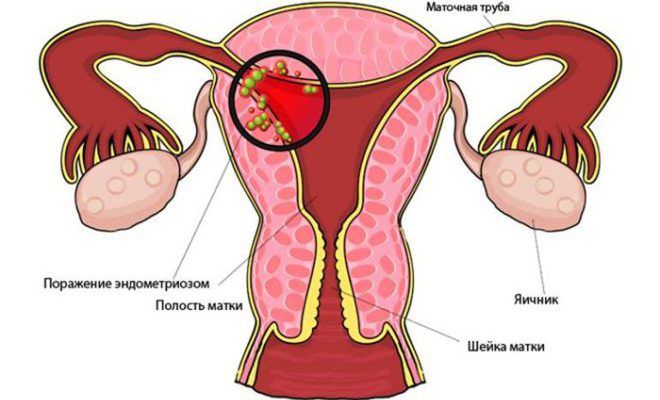
The most common cause the appearance of endometriosis is a medical abortion. For this reason, women should protect themselves in advance with high quality before each sexual intercourse, so that pregnancy does not occur. There are many medicines and other medicines, but in practice, the Mirena spiral with endometriosis has proven itself from the best side.
This medical invention can solve two problems:
- have a therapeutic effect during the fight against female endometriosis;
- provide contraception during intercourse.
The spirals under consideration are capable of functioning for about 4–5 years. Much depends on the choice of treatment being considered. The highly effective action of the spiral, which is installed in the uterus during endometriosis, is time-tested and will not cause any discomfort to women during the period of use. Based on the reviews of many experts, taking into account the results (reviews and results are mostly positive), there is a slowdown in the growth of fibroids in the foci of endometriosis.
How does an intrauterine device work?
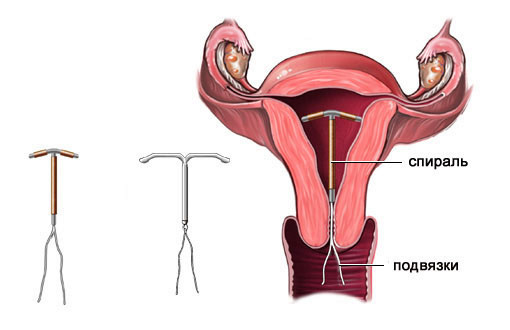
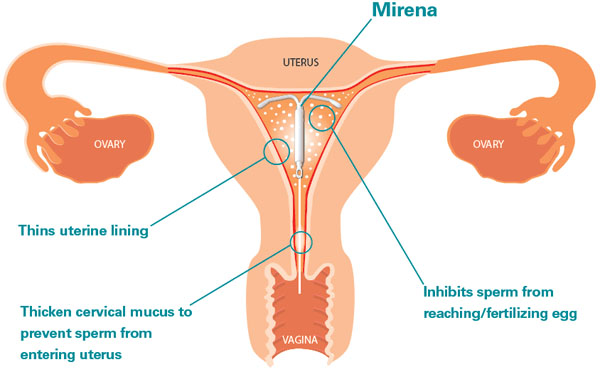
Mirena, is a kind of product, consisting of a special composition, which through the shell can act in the form hormonal contraceptive, it has a small dose of a substance such as Levonorgestrel. It is able to be released and act as intended within female body. The coil design is inserted into the inside of the uterus.
The main actions of the spiral:
- reduces bleeding in the endometrial region;
- inhibits the activity of spermatozoa that have entered the uterus;
- thins the inner uterine surface, which causes menstruation of a meager type (their complete absence);
- reduces the function of the corpus luteum;
- inhibits ovulation processes;
- changes the intensity of patency of cervical mucus;
- changes the hormonal balance and suppresses the estrogenic activity that prevails during the development of endometriosis.
The Mirena spiral when detecting endometriosis in women is reliable contraception, as well as a means for quality preventive measures. Based on the reviews of doctors and many specialists, a positive opinion was received from patients who practiced this method of treatment.
Endometriosis and its correction
Depending on what manifestations of the disease were found in patients, treatment is chosen. He is selected by the doctor, having made preliminary examinations.
Correction of endometriosis occurs in different ways:
- application surgical operations associated with the removal of foci of pathology;
- with the help of certain hormones, the woman's body is introduced into a state of artificial menopause;
- assigned use oral contraceptives, as well as peculiar additives;
- The most effective correction method is the Mirena coil.
In any case, after choosing, it is important to carefully monitor the state of the body and its reaction. If the condition worsens, it is necessary to report this to specialists who know what to do.
When can Mirena be used?
In addition to the fact that a synthetic agent creates reliable protection against conception, it also has a therapeutic and prophylactic effect in the presence of:
- uterine bleeding during premenopause;
- strong and prolonged menstruation with signs of anemia;
- the occurrence of a polyp in the area of \u200b\u200bthe inner part of the uterus;
- endometrial hyperplasia;
- uterine leiomyoma;
- pathology of the cervical canals during the postoperative period;
- endometrioid pathology.
Taking into account the opinion of the patient, the need to use the spiral is prescribed exclusively by the gynecologist, but before everything, he must conduct the necessary examinations and tests to take into account possible complications.
Mirena is produced by well-known specialists - it is a German pharmaceutical company named Bayer. The design feature of the spiral is that it has a hormonal-elastomer core located in a certain body. It is coated with a shell, which is responsible for regulating the release of active substances.
Doctors and experts say that the Mirena spiral is installed in the woman's uterus painlessly, and its effect (contraception) occurs almost from the day of installation. The peculiarity of the case is that it has a loop in a certain place, a thread is attached there. It is used when extracting the spiral system. According to experts, the design feature makes it possible to carry out safe installation and removal.
Doctors consider the advantage of the helix over the pill is that the helix does not work through the gastrointestinal tract. This eliminates many side effects and the best result.
The coil is placed inside the uterus. There, the corresponding hormones, including progesterone, are released. The effectiveness of the system under consideration lies in the fact that it is able to have a local effect, for this reason a lower level of hormones is required from this system.
The actions of active substances are aimed at suppressing growths in the structure of the endometrium. During the presence of the structure in the uterus, the following occurs:
- reduction of pain symptoms;
- reducing the intensity of bleeding, or stopping them;
- reduction in the duration of menstruation;
- regulation of the menstrual cycle.
If the form of endometriosis is mild, then the healing process begins almost immediately. It is worth knowing that after placing the spiral in the uterus, there may be slight bleeding, but after a certain time they will pass. If this does not happen, then it is important to contact specialists.
release of the required hormone
Reviews of medical specialists about the Mirena spiral for endometriosis in most cases are satisfactory. It is used in the treatment of illness. In connection with the release of progesterone by Mirena, the source of the disease begins to regress. It happens that reverse processes of transformation of pathology begin.
It is important to remember that the focus of the disease in question is able to develop and progress depending on the effects of a substance such as estrogen. For this reason, it is recommended to use Mirena for endometriosis and only her, since the composition active substance quite suitable for the treatment of the disease. An exception is the situation that is associated with pregnancy planning, the presence of cancerous tumors or inflammation.
Contraindications
- against the background of any present infectious diseases of the female body;
- if there is a possibility of pregnancy;
- if present genital infection or gynecological inflammation;
- if there is uterine bleeding with an incomprehensible focus;
- if there are genetic or acquired deformation problems with the uterus and its inner surface;
- in case of suspicion malignant tumors in any location of the woman's body.
Diagnostic steps before introducing Mirena
- conduct an ultrasound of the genital organs of a woman;
- test for chronic infections;
- examine the cervical canal (perform bacterial cultures);
- examination of smears for the presence of possible infectious foci.
The considered methods can help to install the spiral without complications in the future.
Duration
The Mirena protection system, as mentioned above, is valid for about 5 years, but it is not forbidden to use it again. There are no negative reviews for this.
A woman will not begin to recover and will not spoil the condition of her figure (these phenomena are observed when using spirals from an unverified manufacturer). Due to the fact that the Mirena spiral, during endometriosis, is able to have a positive effect, and the body during treatment is not overwhelmed by the concentration of a hormone such as progesterone.
After the system is installed inside the uterus, the fair sex will no longer need to carry out careful contraceptive actions for a set period of time.
How does Mirena affect the intensity of intrauterine bleeding?
When treated with the Mirena coil, women may notice that their periods become less intense, or absent altogether. This causes additional unrest, but according to many gynecologists and doctors, there is no reason to worry. It is worth knowing that when using the Mirena coil during treatment, such outcomes are considered positive.
![]()
In the event of a complete cessation of menstruation, women taking spiral treatment are advised to consult a doctor so that specialists can make an adjustment. If no manifestations occur, there is no pain, there is no bleeding, then this means that endometriosis is receding.
Due to the properties of the substance that is in the spiral, the fair sex suppresses ovulation and the growth of the endometrial layer. Every month, women experience a decrease in discharge, bleeding, and pain scores.
Are there any side effects when using Mirena?
The use of the Mirena spiral for endometriosis of the uterus has its pros and cons, as well as medications. During treatment, a woman may experience the following effects:
- frequent vomiting that does not bring relief;
- the appearance of neoplasms that are active on the surface of the ovaries after the installation of the spiral;
- the body weight index in women changes, some time after the appointment of treatment;
- the appearance of symptoms of unexplained nausea;
- the color of the skin cover changes noticeably, the fact that acne may occur is not excluded;
- increased sensitivity of the mammary glands, it happens that the pain manifests itself too brightly;
- manifestation of frequent dizziness;
- the occurrence of inexplicable weakness after the installation of the spiral;
- headache with prolonged gag reflexes.

If there are any reactions to the use of Mirena, as described above, it is worthwhile to quickly inform the specialists about the occurring symptoms. Depending on how the side effect is expressed, specialists will decide on the future treatment of endometriosis with a spiral.
What do experts think about the Mirena spiral?
Mirena, during endometriosis, has mostly positive reviews. During treatment, based on the statistics kept by doctors, in many women the pain in the lower abdomen disappears after a week, and spotting disappears after the first cycle.
The occurrence of side effects is rare. If a woman has thrombosis, or cancer, then it is important not to start treatment with the spiral in question.
With regards to positive effects, it is worth noting that the use of a spiral for endometriosis lowers the risk of inflammation in the pelvic area. It is worth remembering that the device is not able to protect the fairer sex from diseases that are transmitted through sexual contact.
Preventive actions
The disease is easier to prevent than to fight it later. Specialists have developed methods that can prevent the development of such a pathology as endometriosis. If you follow the advice of doctors, then there will be fewer problems.

During prevention, it is worth observing certain rules:
- fight against weight gain;
- reduction of psychological stress;
- refusal to perform abortions;
- timely treatment of gynecological diseases;
- regular visits to the gynecologist.
During preventive actions with the help of the Mirena coil, it is important to lead an active lifestyle. Today, there is a lot of information that moderate physical activity in the form of exercise can significantly reduce estrogen levels. This is how the “erection of a barrier” for the possible processes of the development of the disease occurs.
It is important to choose the optimal complex exercise that you can do every day. During endometriosis, it is important to stop using tampons. These hygiene practices can interfere with the passage of natural blood flow. This provokes the reflux of blood into the region of the endometrium, as well as into the region of the fallopian tubes.
It is worth paying attention to daily nutrition. It should be full and saturated with high-quality vegetables and fruits. It is recommended not to drink a lot of coffee or carbonated drinks.
It is possible and necessary to fight endometriosis. If you start prevention with the help of a spiral, then experts predict the treatment will be favorable and without complications. Otherwise, the consequences are unfortunate.
Why do most gynecologists prescribe IUDs so often in the treatment of "female" diseases? Which spiral occupies a leading position in the positive feedback from patients? And most importantly, is it possible to put a spiral with uterine myoma? You will find comprehensive answers to these and other questions regarding dangerous diseases of the pelvic organs in our article.
Myoma is a benign tumor caused by the growth of myometrial cells in different layers of the uterine wall. The number of myocytes can be any, the location is also different.
The main causes of the disease are considered to be:
- hormonal failure in a woman's body;
- disruption of the endocrine system;
- artificial termination of pregnancy;
- obesity;
- heredity;
- long stay in stressful situations.
On the early stages detection of the disease, surgery is usually not required. Therefore, it is better to scheduled inspection at the gynecologist at least once every six months. As a rule, the disease develops asymptomatically, you will not be able to determine its presence on your own.

Due to the fact that women over the age of 30 are at risk of developing the disease, it is important to choose the right method of protection against unwanted pregnancy. If you are sexually active and have sex almost every day, choose reliable and safe contraception so that it is certainly combined with the treatment of the disease. There are many treatment options for uterine fibroids. But it was the use of spirals that gained a huge advantage over other methods.
Types of Navy. How to make the right choice?
Peak of popularity intrauterine devices came in the 70s, when contraceptives containing copper, silver and gold first appeared. The use of this method has been widely used by physicians. But the feedback from the patients at that time was negative, because often the usual spirals caused bleeding and pain during the menstrual cycle.
Soon the world was covered by a new wave of hormonal IUDs, which were highly effective and safe to use. They stopped calling those Negative consequences, which were noted earlier, but, on the contrary, helped to regulate the cycle. Therefore, gynecologists began to prescribe this type of contraception to women with diseases of the reproductive organs.
What does a spiral look like, and what is the mechanism of its action?
Mirena has a T-shape, like most other spirals, that is, it resembles anatomical structure uterus. In its structure there is a special compartment for the hormone levonorgestrel.
Every day it is released into the uterine cavity through a special septum. The spiral acts gently, and is suitable for many women as a contraceptive, as well as the prevention of diseases of the pelvic organs.
What is the main difference between Mirena and other types of IUDs?
The Mirena spiral for uterine fibroids is prescribed by doctors around the world for a reason. This popularity is primarily due to a number of factors that have a beneficial effect on the disease. Let's take a look at them:
- The Mirena coil is last generation hormonal IUDs.
- It contains levonorgestrel, a hormone that compensates for the deficiency of progesterone, due to the low concentration of which problems begin in the uterus.
- The active substance has an exclusively local effect on the endometrium, almost without getting into the bloodstream, due to which it is achieved best effect. The development of the disease stops.
- Painful sensations gradually disappear.
- Bleeding between cycles decreases or disappears altogether.
- Menstruation becomes scarce, and in some women it stops for the entire period of use.
- Normalized hormonal background, as a result of which the neoplasm stops its development.
- The spiral is effective for the prevention of concomitant uterine endometriosis.
- Allowed for chronic diseases such as diabetes and hypertension.
- Installation for as much as 5 years gives a long-term effect on myoma, and, therefore, stops the growth of myomatous nodes.
- Cancels surgical removal of the uterus.
- Does not affect the functioning of the ovaries.
What do the patients themselves say about the spiral?
Mirena for uterine fibroids received great amount positive feedback from patients. It is safe and effective for most women. The use of this method of contraception is allowed even for nursing mothers, which once again confirms the guarantee of the absence of side effects.

Here is what women who have already tried this drug say:
“My Mirena has been standing for 3 years, and all this time I have been very satisfied. My doctor prescribed this method of contraception after the discovery that I had fibroids and fibromyomas at 7-8 weeks. I'm tired of heavy and prolonged periods. On this occasion, I went to the hospital several times, where they did scraping to stop the bleeding. After the Mirena was put in, the problems ended. Menstruation stabilized, pain disappeared at the beginning of the cycle in the lower abdomen and lumbar region. I can lead an active lifestyle, from which I have already managed to wean - I swim, run, sign up for fitness. No problems with sex, libido even increased. With her husband, she began to reach orgasm more often. The gynecologist said that the spiral is only 5 years old. In a couple of years I will take it off so that the uterus can rest. Then again I want to put, and live normal life feel like a normal woman." Kseniya.
“My sister had this coil put in 3 years ago. And everything is fine. Stop doing nonsense, read nasty things on forums, collect bad experience, think about your health, because you have one! Better trust your doctor!" Oksana.
“Mirena is a healing coil, she saved my sister from a hysterectomy. And, if our doctor says to put more, we will pay and we will definitely do it. Yes, it's not cheap, but the result is worth it. Marina.
Every woman should remember that, despite all the positive reviews, with uterine myoma, the installation of the Mirena spiral does not give a 100% result. However, its use will undoubtedly have a positive effect, stop the development of the tumor and improve the condition of the body as a whole.
What contraindications and side effects do you need to know about when choosing a Mirena coil?

Naturally, the installation of this method of contraception is possible only by a qualified specialist, after passing a full examination, passing all the necessary tests and confirming the diagnosis. Examination and observation of a patient with a Mirena coil installed with a diagnosed uterine myoma is repeated after a month. Then you will need to see a doctor in 3 months, and again in a year.
Contraindications for this method of contraception:
- Individual sensitivity to action active substance.
- Breast cancer.
- Malignant neoplasms.
- Pregnancy.
- Liver disease.
- Uterine bleeding of unknown nature.
- Infectious diseases of the genital organs.
- Deep vein thrombosis.
- Within a month after giving birth.
- Acute inflammation of the pelvic organs.
- Cervicitis.
Possible side effects:
- increase in days of menstrual bleeding;
- painful periods (usually in the first months of use);
- highlighting a smearing character during the cycle;
- cysts in the ovaries (often go away on their own).
If you have found at least one of the items on the list, consult with your doctor about a possible alternative treatment. Each patient needs an individual examination and approach to the choice of hormonal therapy.
Spiral installation features
The procedure for installing Mirena is carried out at the end of menstrual bleeding. The uterine cavity is checked for pregnancy, infection, and its exact shape and size is determined. Manipulation is painless, and the entire installation procedure takes no more than 30 minutes. To avoid complications, you should refrain from sexual intercourse for several weeks after the operation.
The positive aspects of the treatment of uterine fibroids with the Mirena spiral:
- safety and efficacy;
- replacement for surgical treatment;
- blocking the growth of the neoplasm;
- protection from unwanted pregnancy;
- lack of weight gain;
- installation without pain;
- additional healing effect with concomitant diseases;
- regulation of the menstrual cycle.
Negative points:
- the high cost of both the spiral and accompanying medical care;
- prolonged addiction;
- possible sensitivity during sex;
- the likelihood of interaction of the hormone with other drugs.
In any case, no matter how expensive the treatment of uterine fibroids is, it is simply necessary. The disease should not be started in any case, otherwise the consequences can be tragic for you. Moreover, it is worth monitoring women's health if you are planning a pregnancy.
The IUD is an excellent way to prevent benign neoplasms, and the Mirena spiral, according to numerous reviews of women, has earned the status of the safest and most effective method treatment, including conditions for uterine fibroids.
intrauterine therapeutic system 20 mcg/24 h: 1 pc. Reg. No.: P N014834/01
Clinico-pharmacological group:
Intrauterine contraceptive
Release form, composition and packaging
Intrauterine Therapy System (IUD) with a release rate of the active substance of 20 μg / 24 h consists of a white or almost white hormonal elastomeric core placed on a T-shaped body and covered with an opaque membrane that regulates the release of levonorgestrel a. The T-body is provided with a loop at one end and two arms at the other; threads are attached to the loop to remove the system. The IUD is placed in the guide tube. The system and the conductor are free from visible impurities.
Excipients: dimethicone (polydimethylsiloxane elastomer) - 52 mg.
1 PC. - sterile blisters made of TYVEK material and polyester (PETG or APET) (1) - cardboard packs.
Description of the active ingredients of the drug Mirena ®»
pharmachologic effect
Mirena ® is an intrauterine therapeutic system (IUD) that releases levonorgestrel and has mainly a local gestagenic effect. The gestagen (levonorgestrel) is released directly into the uterine cavity, which allows it to be used in extremely low daily dose. High concentrations of levonorgestrel in the endometrium contribute to a decrease in the sensitivity of its estrogen and progesterone receptors, making the endometrium immune to estradiol and exerting a strong antiproliferative effect. When Mirena ® is used, morphological changes in the endometrium and a weak local reaction to the presence of a foreign body in the uterus are observed. Thickening of the mucous membrane of the cervical canal prevents the penetration of sperm into the uterus. Mirena ® prevents fertilization due to inhibition of sperm motility and function in the uterus and fallopian tubes. Some women also experience suppression of ovulation.
Previous use of the drug Mirena ® does not affect the childbearing function. Approximately 80% of women who want to have a baby become pregnant within 12 months after the IUD is removed.
In the first months of using the drug Mirena ® , due to the process of inhibition of endometrial proliferation, there may be an initial increase in spotting bloody discharge from the vagina. Following this, a pronounced suppression of endometrial proliferation leads to a decrease in the duration and volume of menstrual bleeding in women using Mirena ®. Scanty bleeding often transforms into oligo- or amenorrhea. At the same time, ovarian function and the concentration of estradiol in the blood plasma remain normal.
Mirena ® can be successfully used for the treatment of idiopathic menorrhagia, i.e. menorrhagia in the absence of hyperplastic processes in the endometrium (endometrial cancer, metastatic uterine lesions, submucosal or large interstitial node of uterine fibroids, leading to deformation of the uterine cavity, adenomyosis), endometritis, extragenital diseases and conditions accompanied by severe hypocoagulation (for example, von Willebrand disease, severe thrombocytopenia ), the symptoms of which are menorrhagia.
After 3 months of using the drug Mirena ® , menstrual blood loss in women with menorrhagia is reduced by 62-94% and by 71-95% after 6 months of use. When using the drug Mirena ® for two years, the effectiveness of the application
drug (reduction of menstrual blood loss) is comparable to surgical methods of treatment (ablation or resection of the endometrium). A less favorable response to treatment is possible with menorrhagia due to submucosal uterine myoma. Reducing menstrual blood loss reduces the risk of iron deficiency anemia. The drug Mirena ® reduces the severity of symptoms of dysmenorrhea. The efficacy of Mirena® in preventing endometrial hyperplasia during chronic estrogen therapy was equally high with both oral and transdermal estrogen.
Indications
- contraception;
- idiopathic menorrhagia;
- prevention of endometrial hyperplasia during replacement therapy estrogen.
Dosing regimen
Mirena ® is injected into the uterine cavity. Efficiency is maintained for 5 years. The release rate of levonorgestrel in vivo at the beginning is approximately 20 μg / day and decreases after 5 years to approximately 10 μg / day. The average rate of release of levonorgestrel is approximately 14 mcg / day for up to 5 years. Mirena can be used in women receiving substitution hormone therapy in combination with oral or transdermal estrogen preparations that do not contain progestogens.
With the correct installation of the drug Mirena ®, carried out in accordance with the instructions for medical use, the Pearl Index (an indicator reflecting the number of pregnancies in 100 women using a contraceptive during the year) is approximately 0.2% over 1 year. The cumulative rate, reflecting the number of pregnancies in 100 women using a contraceptive for 5 years, is 0.7%.
For the purpose of contraceptionwomen of childbearing age Mirena ® should be placed in the uterine cavity within 7 days from the onset of menstruation. Mirena ® can be replaced with a new IUD on any day of the menstrual cycle. An IUD can also be inserted immediately after an abortion in the first trimester of pregnancy.
After childbirth the installation of the IUD should be carried out when the uterus involutes, but not earlier than 6 weeks after birth. With prolonged subinvolution, it is necessary to exclude postpartum endometritis and postpone the decision to introduce Mirena until the involution is completed. In case of difficulty in inserting the IUD and / or very severe pain or bleeding during or after the procedure, physical examination and ultrasound should be performed promptly to rule out perforation .
To protect the endometrium during estrogen replacement therapy in women with amenorrhea, Mirena ® can be installed at any time; in women with preserved menstruation, the installation is performed in the last days of menstrual bleeding or withdrawal bleeding.
Mirena should not be used for postcoital contraception.
Rules for the use of the Navy
Mirena ® is supplied in a sterile package, which is opened only immediately before the installation of the IUD. Asepsis must be observed when handling an opened system. If the sterility of the packaging appears to be compromised, the IUD should be disposed of as medical waste. You should also handle the IUD removed from the uterus, as it contains hormone residues.
Insertion, removal and replacement of the IUD
Before installation Mirena ® woman should be informed about the effectiveness, risks and side effects this Navy. It is necessary to conduct a general and gynecological examination, including an examination of the pelvic organs and mammary glands, as well as an examination of a smear from the cervix. Pregnancy and sexually transmitted diseases should be excluded, and genital infections should be completely cured. Determine the position of the uterus and the size of its cavity. Particularly important is the correct location of the drug Mirena ® in the bottom of the uterus, which ensures a uniform effect of the progestogen on the endometrium, prevents the expulsion of the IUD and creates conditions for its maximum effectiveness. Therefore, you should carefully follow the instructions for installing Mirena ®. Since the technique of insertion in the uterus of different IUDs is different, Special attention you should pay attention to working out the correct technique for installing a particular system.
The woman should be re-examined 4-12 weeks after insertion, and then once a year or more often if clinically indicated.
Before installation the drug Mirena ®, pathological processes in the endometrium should be excluded, since irregular bleeding / spotting is often noted in the first months of its use. Also, pathological processes in the endometrium should be excluded if bleeding occurs after the start of estrogen replacement therapy in a woman who continues to use Mirena ®, previously established for contraception. Appropriate diagnostic measures should also be taken when irregular bleeding develops during long-term treatment.
Mirena ® preparation delete by gently pulling on the threads grasped by forceps. If the threads are not visible and the system is in the uterine cavity, it can be removed using a traction hook to remove the IUD. This may require the expansion of the cervical canal.
The system should be removed 5 years after installation. If a woman wants to continue using the same method, a new system can be installed immediately after the previous one is removed.
If further contraception is needed, in women of childbearing age, removal of the IUD should be performed during menstruation, provided that the menstrual cycle is preserved. If a system is removed in the middle of a cycle and a woman has had sexual intercourse within the previous week, she is at risk of becoming pregnant, unless the new system was installed immediately after the old one was removed.
The insertion and removal of an IUD may be accompanied by certain painful sensations and bleeding. The procedure may cause syncope due to a vasovagal reaction or a seizure in patients with epilepsy.
After removal of the drug Mirena ®, the system should be checked for integrity. In case of difficulties with the removal of the IUD, isolated cases of slipping of the hormonal-elastomer core on the horizontal arms of the T-shaped body were noted, as a result of which they were hidden inside the core. Once the integrity of the IUD is confirmed, this situation does not require additional intervention. Limiters on the horizontal arms usually prevent the core from completely separating from the T-body.
Instructions for the introduction of the IUD
It is installed only by a doctor using sterile instruments.
Mirena ® is supplied with a guidewire in a sterile package that must not be opened prior to installation.
Do not re-sterilize. For single use only. Do not use Mirena ® if the inner packaging is damaged or open. Do not install Mirena ® after the expiration of the month and year indicated on the package.
Before installation, you should read the information on the use of Mirena ®.
Preparation for the introduction
1. Conduct a gynecological examination to determine the size and position of the uterus and to rule out any signs of acute genital infections, pregnancy or other gynecological contraindications for Mirena ® placement.
2. Visualize the cervix with speculums and completely clean the cervix and vagina with a suitable antiseptic solution.
3. If necessary, use the help of an assistant.
4. Grab upper lip cervix with forceps. Straighten the cervical canal by gentle traction with forceps. The forceps must be in this position during the entire time of insertion of Mirena ® to ensure gentle traction of the cervix towards the inserted instrument.
5. Carefully advancing the uterine probe through the cavity to the bottom of the uterus, determine the direction of the cervical canal and the depth of the uterine cavity (the distance from the external os to the bottom of the uterus), exclude septa in the uterine cavity, synechia and submucosal fibroma. If the cervical canal is too narrow, widening of the canal is recommended and pain medication/paracervical block may be used.
Introduction
1. Open the sterile package. After that, all manipulations should be carried out using sterile instruments and sterile gloves.
2. Move the slider forward in the direction to the farthest position in order to draw the IUD into the guide tube.
Do not move the slider downwards as this may lead to premature release of Mirena ® . If this happens, the system will not be able to be placed inside the conductor again.
3. While holding the slider in the farthest position, set the upper edge of the index ring in accordance with the measured probe distance from the external os to the fundus of the uterus.
4. While continuing to hold the slider in its furthest position, advance the guidewire gently through the cervical canal and into the uterus until the index ring is about 1.5-2 cm from the cervix.
Do not force the guidewire. If necessary, expand the cervical canal.
5. Keeping the guidewire motionless, move the slider to the mark to open the horizontal shoulders of the Mirena ® preparation. Wait 5-10 seconds until the horizontal hangers are fully opened.
6. Gently push the guidewire inward until the index ring contacts the cervix. Mirena ® should now be in the fundal position.
7. Holding the conductor in the same position, release Mirena ® by moving the slider as far down as possible. While holding the slider in the same position, carefully remove the conductor by pulling on it. Cut the threads so that their length is 2-3 cm from the external os of the uterus.
If you have any doubts that the system is installed correctly, check the position of the Mirena ® preparation, for example, using ultrasound or, if necessary, remove the system and insert a new, sterile system. Remove the system if it is not completely in the uterine cavity. The remote system must not be reused.
Removal/replacement of Mirena ®
Before removing/replacing Mirena ® , you should read the instructions for use of Mirena ® .
The Mirena ® preparation is removed by gently pulling on the threads grasped by the forceps.
The doctor can set new system Mirena ® immediately after removal of the old one.
Side effect
In most women, after the installation of the drug Mirena ®, there is a change in the nature of cyclic bleeding. AT
during the first 90 days of using Mirena ®, an increase in the duration of bleeding is noted by 22% of women, and irregular bleeding occurs in 67% of women, the frequency of these phenomena decreases to 3% and 19%, respectively, by the end of the first year of its use. At the same time, amenorrhea develops in 0%, and rare bleeding in 11% of patients during the first 90 days of use. By the end of the first year of use, the frequency of these phenomena increases to 16% and 57%, respectively.
When using the drug Mirena ® in combination with long-term estrogen replacement therapy, in most women during the first year of use, cyclic bleeding gradually stops.
The following are data on the incidence of adverse drug reactions that have been reported with the use of Mirena ®. According to the frequency of occurrence, adverse reactions (HP) are divided into very frequent (≥1/10), frequent (from ≥1/100 to< 1/10), нечастые (от ≥1/1000 до < 1/100), редкие (от ≥1/10 000 до < 1/1000) и с неизвестной частотой. HP представлены по классам системы органов согласно MedDRA . Данные по частоте отражают приблизительную частоту возникновения HP, зарегистрированных в ходе клинических исследований препарата Мирена ® по показаниям "Контрацепция" и "Идиопатическая меноррагия" с участием 5091 женщин.
HP, which were reported during clinical trials of the drug Mirena ® for the indication "Prevention of endometrial hyperplasia during estrogen replacement therapy" (involving 514 women), were observed with the same frequency, except in cases indicated by footnotes (*, **).
From the immune system: the frequency is unknown - hypersensitivity to the drug or components of the drug, including rash, urticaria and angioedema.
Mental disorders: often - depressed mood, depression.
From the side nervous system: very often - headache; often migraine.
From the side digestive system: very often - abdominal pain / pain in the pelvic area; often - nausea.
From the skin and subcutaneous tissues: often - acne, hirsutism; infrequently - alopecia.
From the musculoskeletal system: often - back pain**.
From the reproductive system and mammary glands: very often - a change in the volume of blood loss, including an increase and decrease in the intensity of bleeding, spotting, oligomenorrhea, amenorrhea, vulvovaginitis, discharge from the genital tract *; often - infections of the pelvic organs, dysmenorrhea, pain in the mammary glands **, expulsion of the IUD (complete or partial), ovarian cysts; rarely - perforation of the uterus.
From the side of the cardiovascular system: frequency unknown - increased blood pressure.
* "often" for the indication "Prevention of endometrial hyperplasia during estrogen replacement therapy."
** "Very common" for the indication "Prevention of endometrial hyperplasia during estrogen replacement therapy".
MedDRA terminology is used in most cases to describe certain reactions, their synonyms, and related conditions.
Additional Information
If a woman with an established Mirena ® drug becomes pregnant, the relative risk of ectopic pregnancy increases.
The partner can feel the threads during intercourse.
The risk of breast cancer when using Mirena ® for the indication "Prevention of hyperplasia
of the endometrium during estrogen replacement therapy" is unknown. Cases of breast cancer have been reported (frequency unknown).
The following HPs have been reported in connection with the insertion or removal of Mirena ® : pain during the procedure, bleeding during the procedure, vasovagal reaction associated with insertion, accompanied by dizziness or fainting. The procedure can provoke an epileptic seizure in patients suffering from epilepsy.
infection
Cases of sepsis (including group A streptococcal sepsis) have been reported following IUD insertion.
Contraindications
- pregnancy or suspicion of it;
— inflammatory diseases pelvic organs (including recurrent);
- infections of the lower parts urinary tract;
— malignant neoplasms uterus or cervix;
- postpartum endometritis;
- septic abortion within the last three months;
- cervicitis;
- cervical dysplasia;
- pathological uterine bleeding of unknown etiology;
- progestogen-dependent tumors, incl. mammary cancer;
- diseases accompanied by increased susceptibility to infections;
- congenital and acquired anomalies of the uterus, incl. fibromyomas leading to deformation of the uterine cavity;
- acute liver disease, liver tumors;
- Hypersensitivity to the components of the drug.
Pregnancy and lactation
The use of the drug Mirena ® is contraindicated in pregnancy or suspicion of it. If pregnancy occurs in a woman during the use of the drug Mirena ®, it is recommended to remove the IUD, because. any intrauterine device left in situ increases the risk of spontaneous abortion and premature birth. Removal of Mirena ® or probing of the uterus may lead to spontaneous abortion. If careful removal of the intrauterine contraceptive is not possible, medical abortion should be discussed. If a woman wishes to continue the pregnancy and the IUD cannot be removed, the patient should be informed of the risks and possible consequences premature birth for a baby. AT similar cases the course of pregnancy should be carefully monitored. An ectopic pregnancy must be ruled out.
The woman should be advised that she should report all symptoms suggestive of pregnancy complications, in particular colicky abdominal pain accompanied by fever.
Due to intrauterine use and local action of the hormone, the possibility of a virilizing effect on the fetus must be taken into account. Due to the high contraceptive efficacy of Mirena ®, clinical experience related to pregnancy outcomes with its use is limited. However, the woman should be informed that at this point in time there is no evidence of congenital effects caused by the use of Mirena ® in cases of continuation of pregnancy until delivery without removal of the IUD.
About 0.1% of a dose of levonorgestrel can enter the child's body during breastfeeding. However, it is unlikely that it poses a risk to the child at doses released into the uterine cavity after the insertion of Mirena ®.
It is believed that the use of the drug Mirena ® 6 weeks after birth does not have a harmful effect on the growth and development of the child. Monotherapy with gestagens does not affect the quantity and quality of breast milk. Rare cases have been reported uterine bleeding in women using Mirena ® during the lactation period.
Fertility
After removal of the drug Mirena ® in women, fertility is restored.
Application for violations of liver function
Contraindicated in acute liver diseases, liver tumors.
special instructions
Mirena ® should be used with caution in women with congenital or acquired valvular heart disease, bearing in mind the risk of septic endocarditis. When inserting or removing an IUD, these patients should be given antibiotics for prophylaxis.
Levonorgestrel in low doses can affect glucose tolerance, and therefore its concentration in blood plasma should be regularly checked in women with diabetes and using Mirena ® . However, as a rule, there is no need to change therapeutic prescriptions in women with diabetes using Mirena ®.
Some manifestations of polyposis or endometrial cancer may be masked by irregular bleeding. In such cases, additional examination is necessary to clarify the diagnosis. Mirena ® is not a first choice drug either for young women who have never been pregnant, or for postmenopausal women with severe uterine atrophy.
Available data indicate that the use of the drug Mirena ® does not increase the risk of developing breast cancer in postmenopausal women under the age of 50 years. Due to the limited data obtained during the study of the drug Mirena ® for the indication "Prevention of endometrial hyperplasia during estrogen replacement therapy", the risk of breast cancer when using the drug Mirena ® according to this indication cannot be confirmed or denied.
Oligo- and amenorrhea
Oligo- and amenorrhea in women of childbearing age develops gradually, in approximately 57% and 16% of cases by the end of the first year of using Mirena ®, respectively. If menstruation is absent within six weeks after the start of the last menstruation, pregnancy should be excluded. Repeat pregnancy tests for amenorrhea are not necessary unless there are other signs of pregnancy.
When Mirena® is used in combination with permanent estrogen replacement therapy, most women gradually develop amenorrhea during the first year.
Pelvic infections
The guidewire helps protect Mirena® from microbial contamination during insertion, and the Mirena® injection device is specifically designed to minimize the risk of infection. It has been established that the presence of multiple sexual partners is a risk factor for infections of the pelvic organs. Pelvic infections can have serious consequences: they can impair fertility and increase the risk of ectopic pregnancy.
As with other gynecological or surgical procedures, severe infection or sepsis (including group A streptococcal sepsis) can develop after IUD insertion, although this is extremely rare. For recurrent endometritis or pelvic infections, or for severe or acute infections resistant to treatment for several days, Mirena ® should be removed. Even in cases where only a few symptoms indicate the possibility of infection, bacteriological examination and monitoring are indicated.
Expulsion
Possible signs of partial or complete expulsion of any IUD are bleeding and pain. However, the prolapse of the IUD can occur unnoticed by the woman, which leads to the termination of the contraceptive action. Partial expulsion may reduce the effectiveness of Mirena ® .
Since the drug Mirena ® reduces menstrual blood loss, its increase may indicate the expulsion of the IUD.
If the position in the uterine cavity is incorrect, the IUD must be removed. At the same time, a new system can be installed.
It is necessary to explain to the woman how to check the threads of Mirena®.
Perforation and penetration
Perforation or penetration of the body or cervix of the IUD is rare, mainly during insertion, and may reduce the effectiveness of Mirena ® . In these cases, the system should be removed. There may be an increased risk of perforation when inserting an IUD after childbirth, during lactation, and in women with a fixed uterine tilt.
Ectopic pregnancy
Women with a history of ectopic pregnancy, tubal surgery, or pelvic infection are at higher risk of ectopic pregnancy. The possibility of ectopic pregnancy should be considered in the case of lower abdominal pain, especially if it is combined with the cessation of menstruation, or when a woman with amenorrhea begins to bleed. The frequency of ectopic pregnancy when using Mirena ® is approximately 0.1% per year. The absolute risk of ectopic pregnancy in women using Mirena ® is low. However, if a woman with an established Mirena ® drug becomes pregnant, the relative likelihood of an ectopic pregnancy is higher.
Loss of threads
If, during a gynecological examination, the threads for removing the IUD cannot be found in the cervical region, pregnancy must be excluded. The threads can be drawn into the uterine cavity or cervical canal and become visible again after the next menstruation. If pregnancy is excluded, the location of the threads can usually be determined using careful probing with an appropriate instrument. If the threads cannot be detected, it is possible that the IUD has been expelled from the uterine cavity. To determine the correct location of the system, you can use ultrasound procedure(ultrasound). In case of its unavailability or failure, X-ray examination is used to determine the localization of the Mirena ® drug.
ovarian cysts
Since the contraceptive effect of the drug Mirena ® is mainly due to its local action, women of childbearing age usually experience ovulatory cycles with ruptured follicles. Sometimes the atresia of the follicles is delayed, and their development can continue. These enlarged follicles are clinically indistinguishable from ovarian cysts. Ovarian cysts have been reported as an adverse reaction in approximately 7% of women using Mirena ®. In most cases, these follicles do not cause any symptoms, although sometimes they are accompanied by pain in the lower abdomen or pain during intercourse. As a rule, ovarian cysts disappear on their own within two to three months of observation. If this does not happen, it is recommended to continue monitoring with ultrasound, as well as carrying out therapeutic and diagnostic measures. In rare cases, it is necessary to resort to surgical intervention.
Influence on the ability to drive vehicles and control mechanisms
Not observed.
Overdose
With this method of application, an overdose is impossible.
drug interaction
Terms of dispensing from pharmacies
The drug is dispensed by prescription.
Terms and conditions of storage
The drug should be stored out of the reach of children, protected from light at a temperature not exceeding 30 ° C. Shelf life - 3 years.
drug interaction
It is possible to increase the metabolism of gestagens with the simultaneous use of substances that are enzyme inducers, especially isoenzymes of the cytochrome P450 system involved in metabolism medicines such as anticonvulsants (eg, phenobarbital, phenytoin, carbamazepine) and agents for treating infections (eg, rifampicin, rifabutin, nevirapine, efavirenz). The effect of these drugs on the effectiveness of the drug Mirena ® is unknown, but it is assumed that it is not significant, since Mirena ® has a mainly local effect.
